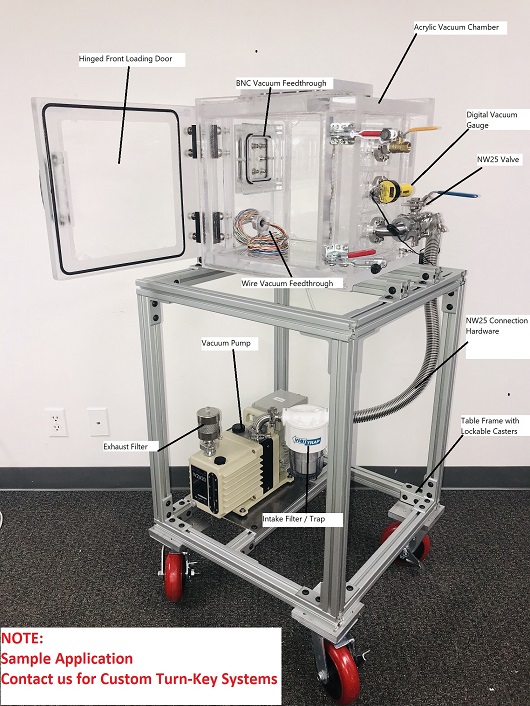
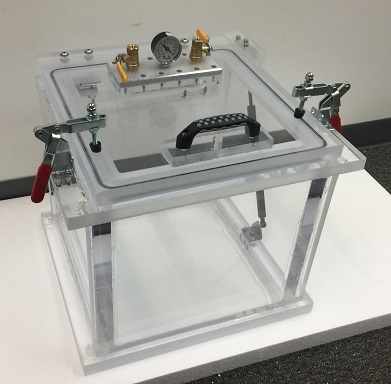
What is the ASTM F2096 Protocol?
The ASTM F2096 protocol serves as a recognized standard for evaluating packaging integrity. This method provides a structured approach for assessing the reliability of sterile barrier systems, ensuring compliance with stringent quality standards.
The procedure requires the operator to create a precise puncture in the test package before fully immersing it beneath one inch of water. Once submerged, controlled internal pressure ranging from 0 to 50mbar (0 to 20 inH20) is introduced into the package. The presence of continuous air bubbles escaping from the packaging material signifies a compromise in structural integrity. This test enables for detection and location of leaks of gross leaks as small as 250 micrometers. After one side is complete, rotate the package by 180 degrees, by facing bottom side up, and retest again.
This is pretty much it.
Achieve excellence in leak detection with ASTM F2096, the benchmark for precision and reliability in bubble leak testing. A comprehensive grasp of this industry-standard protocol is essential for ensuring accurate evaluations and maintaining the highest quality standards. Do not leave compliance to chance. Secure the official documentation today and reinforce the integrity of your testing procedures with absolute confidence. Purchase your Protocol Copy Today, at:
Understanding the Drawbacks of ASTM F2096 for Leak Detection
A significant limitation of this testing method, often overlooked in discussions, is the considerable amount of time required for execution. The process demands meticulous preparation before testing can even begin. The package must first be conditioned and then punctured with precision. This puncturing step, while essential, introduces the possibility of unintended structural compromise, which can impact test reliability.
Once the package has been properly prepared, it must remain fully submerged beneath one inch of water throughout the evaluation. Inflation follows, requiring the operator to maintain vigilance while carefully observing for the emergence of air bubbles that signify a leakage point. Given the subjective nature of this assessment, the technician conducting the test must be fully focused, attentive, and in a proper state of mind (not high) to ensure accurate detection. This process presents additional complications when evaluating elongated or irregularly shaped packages, where ensuring even submersion and complete visibility can be particularly demanding.
Upon completion of the first phase, the test specimen must be repositioned to assess the opposite side, adding yet another layer of complexity. When the procedure concludes, the package must be extracted from the water, which poses an additional logistical challenge. Larger packages, especially those with extended dimensions, retain a significant volume of residual water, leading to excessive dripping upon removal. This necessitates an effective drainage system and a flooring solution designed to mitigate slipping hazards to maintain a safe working environment.
Beyond these procedural challenges, the destructive nature of this method means that tested specimens cannot be repurposed or salvaged. Each package subjected to evaluation must be discarded, increasing material consumption and waste generation. While effective in detecting leaks with high accuracy, the test presents a set of operational considerations that must be weighed carefully against its benefits.
Can the ASTM F2096 Method Be Adapted for Alternative Applications?
Absolutely, this methodology possesses remarkable versatility and has been successfully modified for a broad spectrum of industries, including industrial manufacturing, aerospace engineering, and automotive component testing. Numerous clients have leveraged tailored variations of this approach to assess structural integrity and leak resistance across diverse applications.
One particular client initially expressed skepticism regarding the adequacy of 20 inH2O pressure for achieving the desired level of sensitivity. It is important to recognize that this pressure equates to approximately 0.72 PSI, which, for their specific requirements, proved insufficient. To address this, we engineered a customized tank and high-pressure system capable of delivering 125 PSI, substantially amplifying the detection sensitivity by a factor of approximately 200. This enhancement transformed the test into a highly effective diagnostic tool for their Unit Under Test (UUT), allowing for exceptionally precise leak identification.
Beyond increasing pressure capacity, we integrated a quick-connect and quick-release hose assembly, a customized UUT holding jig, and a streamlined push-button activation system that facilitated seamless pressurization at the exact moment the test was initiated. These enhancements not only increased operational efficiency but also ensured repeatability and accuracy in their evaluations.
It becomes evident that this protocol offers substantial flexibility and can be adapted beyond its original specifications. Variables such as applied pressure, depth of submersion, and the category of unit under examination can be adjusted to meet the specific demands of a given application. The ability to tailor these parameters demonstrates the scalability and adaptability of this methodology, making it a valuable asset across multiple industries.
Conclusion
The ASTM F2096 Bubble Test remains a pillar of reliability in medical packaging integrity assessment, providing a highly precise and effective means of identifying structural weaknesses that could compromise sterility. By submerging a pressurized package in a controlled water environment, this method enables accurate detection of leaks through the observation of escaping air bubbles, ensuring that even the smallest breaches, down to 250 micrometers, are promptly identified.
This technique proves especially advantageous for complex, irregular, or flexible packaging configurations, where traditional vacuum-based methods may fall short. Its effectiveness is heavily reliant on precise pressure calibration, meticulous procedural execution, and a rigorous commitment to documentation and quality control. When applied correctly, this methodology prevents false positives, eliminates oversight, and guarantees compliance with the most stringent regulatory standards.
For companies dedicated to ensuring product sterility and maintaining the highest industry benchmarks, mastering this testing process is non-negotiable. Whether you are looking to optimize your leak detection procedures, enhance compliance, or improve packaging validation, our team is here to provide expert guidance and tailored solutions.
When it comes to medical package integrity, it cannot be left to change, take a look at our Internal Pressurization ASTM F2096 Leak Testing Systems
Or, CONTACT US now to strengthen your packaging integrity and safeguard your products with ASTM F2096 Compliant Testing Instrumentation!
We have more resources with regards to the vacuum bubble leak test that we invite you to read.
Vacuum Bubble Leak Test, A Comprehensive GuideBubble Leak Testing using an acrylic vacuum chamber
See our Vacuum Bubble Emission ASTM 3078 Leak Testing Systems